Atrial fibrillation caused by autoimmunity
“Autoimmune diseases (ADs) affect approximately 10% of the world's population. Because ADs are frequently systemic disorders, cardiac involvement is common…In Cardiac Arrhythmias in Autoimmune Diseases we focus on typical arrhythmias and their pathogenesis…among selected ADs (sarcoidosis, systemic lupus erythematosus, scleroderma, type 1 diabetes, Graves' disease, rheumatoid arthritis, ankylosing spondylitis [AS], psoriasis, celiac disease [CD], and inflammatory bowel disease [IBD]).”
Autoimmune diseases and new-onset atrial fibrillation: a UK Biobank study:
“(Systemic) inflammation is suggested to be associated with atrial fibrillation development. Autoimmune diseases can be used to assess underlying (sub)clinical inflammation.”
“Various autoimmune diseases targeting different organs showed associations with new-onset AF in the general population.
Autoimmune diseases are significantly associated with the risk of new-onset AF in this prospective population-based study, comprising almost half a million participants.
Inflammation may underlie atrial remodeling. Autoimmune diseases, related to increased systemic inflammation, may therefore be associated with new-onset AF…”
“Current main findings of “paradoxical” lower total and LDL-cholesterol levels…strongly support the hypothesis that autoimmune activation is the mechanism underlying the development of persistent AF.”
“The requirement of substantial fibrosis of the right atrium may well be a consequence of autoimmune activation...”
Elicited findings support the notion of enhanced proinflammatory state coupled with an autoimmune process in which apoB, presumably aggregated to SHBG, emerge as a basic mechanism in the development of AF…this is alike the dynamics for type-2 diabetes and inflammatory rheumatic disease.”
Inflammation and its associated immune response are involved in the initiation and maintenance of atrial fibrillation (AF)
“Inflammatory pathways contribute to both electrical and structural atrial remodelling and thrombogenesis in patients with AF.
…the presence of inflammation in the heart or systemic circulation can predict the onset of AF and recurrence…after cardiac surgery, cardioversion, and catheter ablation.
…thromboembolism, a detrimental complication of AF, is also associated with inflammatory activity.”
“…any disease with increased association with inflammation could be a trigger for increased CV risk including arrhythmias, especially highly prevalent AF.”
“…a significant role played by inflammation in the occurrence and continuation of AF, and inflammatory markers such as CRP…and IL-6 are associated with AF, failure of cardioversion, and associated thrombogenesis.29 …higher baseline CRP levels in the patients with more AF recurrence postablation.30 …increased CRP levels associated with recurrence of AF.31 …increased markers of inflammation such as IL-6 and CRP associated with increased AF in the general population…”
…insulin resistance, a major risk factor for CV disease.11 The metabolic syndrome (MetS) is associated with increased pro-inflammatory cytokines…There is much evidence for an association of periodontal disease with increased CV risk with atherosclerosis triggered by resultant systemic inflammation and endothelial dysfunction.18
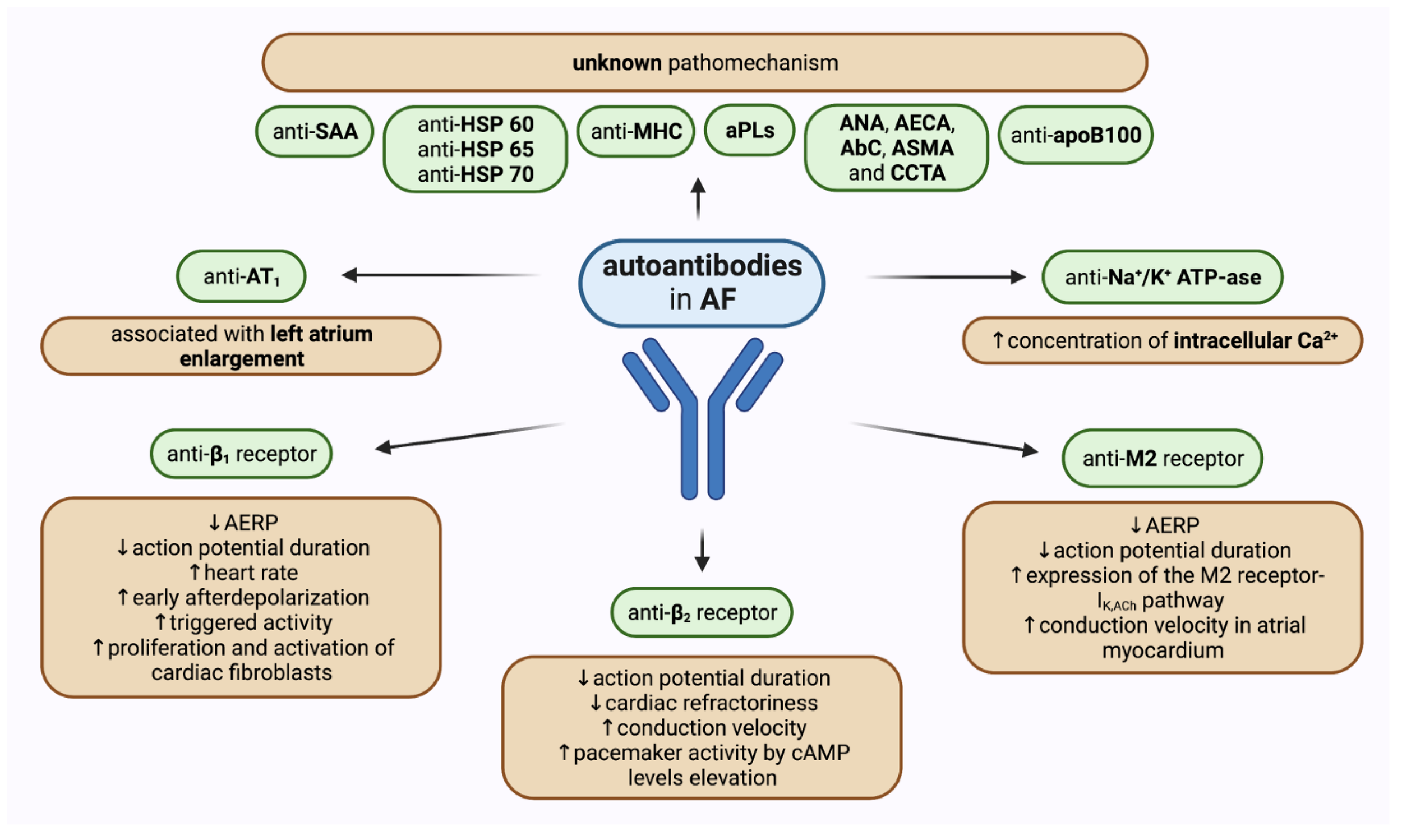
“The time has come to acknowledge autoimmune cardiac arrhythmias as a distinct disease entity. Establishing the autoantibody profile of patients will help to develop novel treatment approaches for patients.”
“Different cardiac receptors and ion channels were identified as targets for autoantibodies, the binding of which either initiates a signaling cascade or serves as a biomarker of underlying remodeling process. Consequently, the wide spectrum of heart rhythm disturbances may emerge, ranging from atrial to ventricular arrhythmias as well as conduction diseases, irrespective of concomitant structural heart disease or manifest autoimmune disorder.”
“Recent studies indicate that autoantibodies play an important role in the development of cardiac arrhythmias, including atrial fibrillation, modulation of autonomic influences on heart rate and rhythm, conduction system abnormalities, and ventricular arrhythmias…there is substantial evidence that autoantibodies play an important role in the development of cardiac arrhythmias in a wide range of disease conditions.”
“Several types of antibodies are associated with atrial fibrillation (Figure 2). The first antibody detected in atrial fibrillation was that against myosin heavy chain 24…. patients with paroxysmal atrial fibrillation…showed the presence of immunoreactivity against cardiac myosin heavy chain...Subsequently, antibodies against the Na/K ATPase 24, 25, the M2-muscarinic cholinergic receptors, the β1 adrenergic receptors, and the heat shock protein (HSP) 65 26…promoting the development of atrial fibrillation...Recent clinical studies have included autoantibodies against β1-adrenergic receptors as an independent variable… 38, 39”
“Autoantibodies known to contribute to the development of bradyarrhythmias (red) and tachyarrhythmias (green) are illustrated here.
The significance of autoantibodies in the pathogenesis of cardiovascular abnormalities …is receiving increasing attention. In particular, there is substantial evidence that autoantibodies play an important role in the development of cardiac arrhythmias in a wide range of disease conditions.”
“There is increasing evidence suggesting that autoimmunity is an important factor in the initiation and perpetuation of AF.”
“Serum concentrations of various autoantibodies have been proven to be significantly increased in patients with different types of AF when compared to healthy people. Most scientific attention has been applied to activating autoantibodies against receptors of the autonomous nervous system. Moreover, a growing number of studies has shown that other autoantibodies may affect pathogenetic pathways leading to AF.”
Noteworthily, not only have concentrations of various antibodies and their correlation with specific types of AF been investigated, but also their predictive values, their correlations with other concomitant heart conditions, and possible pathophysiological mechanisms that determine their impact on atrial structural and electrophysiological change
“(AF)…onset and maintenance requires the presence of an arrhythmogenic substrate that predisposes the patient for risk of these types of arrhythmias and the occurrence of a trigger event.”
“A major characteristic of AF-related structural remodelling is atrial fibrosis, a process closely related to inflammation…
A vicious cycle exists in which inflammation leads to a higher prevalence of structural cardiovascular disease, which in turn leads to more inflammation and AF; in fact, inflammation is known to affect signalling pathways that lead to the development of AF.
Therapy must first target systemic inflammation, since decreasing the inflammatory burden has consistently shown to positively ameliorate the prognosis.
New mapping techniques allowing the characterization of the arrhythmic substrate have opened new perspectives and may help in the treatment of AF in these patients, since atrial tissue is the target of inflammation-induced arrhythmic alterations.”
“Our results showed that psoriasis is significantly associated with an increased risk of developing atrial fibrillation.”
“Several epidemiological studies have shown that psoriasis increases the risk of developing atrial fibrillation…
In subgroup analysis, the greater risk was found in studies from North America, RR 1.482 (95% CI: 1.119-1.964, P < 0.05), whereas a moderate risk was observed in studies from Europe RR 1.43 (95% CI: 1.269-1.628, P < 0.0001)…
Therefore, physicians should monitor patient's physical condition on a timely basis.”
“Autoimmune vasculitis is significantly associated with AF and independently confers worse survival. These observations may represent one mechanism linking autoimmunity and inflammation to the pathogenesis and prognosis of AF.”
“This large‐scale population‐based study is the first to analyze the role of autoimmune vasculitis in the development and prognosis of atrial fibrillation (AF).
…autoimmune vasculitis was significantly associated with incident AF cases compared with non‐AF controls.
AF was more strongly associated with overall mortality in the presence than the absence of autoimmune vasculitis.
…a preexisting autoimmune process may be a potential mechanistic link to the development of new‐onset AF.”
“A significant association between celiac disease and risk of atrial fibrillation was reported in this study.”
“There is a 38% increased risk of atrial fibrillation in patients with celiac disease as compared to individuals without celiac disease used as controls.
Early identification, lifestyle modification, adherence and compliance to gluten-free diet, could slow the risk of AF…”
Inflammation and oxidative stress have been found to be responsible of many molecular mechanisms of CD including activation of immune cells such as macrophages, T and B cells, neutrophils and inflammatory cytokines (IL-6, TNF-α). These cytokines and activated immune cells could affect the contractility and electrical myocytes stability inducing fibroblast activation and cellular fibrosis [24-26]. These atrial changes provide reentrant arrhythmias confirmed clinically and electrocardiogram [27].
This study would be considered global research because it includes countries around the world such as USA, UK, Sweden, and others.”
“Our results indicate that patients with coeliac disease, verified by intestinal biopsy, are at increased risk of atrial fibrillation…
Adjustment for hypertension did not alter the HR for AF and hypertension therefore seems unlikely to explain the increased risk of AF in CD.”
“Our findings support a role of autoimmune disease in the pathophysiology of AF, potentially acting through systemic inflammation, which has consistently been linked to AF risk.
More profound systemic inflammation (at the time of diagnosis before a gluten-free diet is introduced reducing the inflammation) could be one interpretation of why the HR is so much higher around the time of diagnosis. We also found a positive association before diagnosis of CD when inflammation caused by undiagnosed CD is likely to have been most intense.”
“The presented findings support that chronic oral inflammations likely affect the pathogenesis of AF by multiple pathways, suggesting the existence of an oral-heart-axis.”
“Chronic oral inflammation induced by bacterial biofilm (gingivitis, periodontitis, and apical endodontic lesions) cause a localized inflammation, which affects cardiac structural and electrophysiological remodeling linked to atrial fibrillation by:
(i) low level bacteremia by which oral bacteria enter the blood stream at inflamed sites of the oral cavity and invade the heart; (ii) systemic inflammation induced by inflammatory mediators which are released from the sites of oral inflammation into the blood stream, affecting ventricular remodeling; (iii) autoimmunity against molecular structures expressed in the heart, such as HSP60/65 and citrullinated cardiac proteins, caused by the host immune response to specific components of oral pathogens;
Make it stand out
(iv) arrhythmogenic effects mediated by activation of the autonomic nervous system during inflammation; (v) effects resulting from specific bacterial proteins and toxins, such Porphyromonas gingivalis PAP and leukotoxin A (LtxA) that are produced by oral pathogenic bacteria and induce the formation of anticitrullinated protein antibodies (ACPA).”
“One of the many responses of the body to an infection is activation of the autonomic nervous system. An important functional component of this system is the inflammatory reflex, a sensory pathway to detect and localize the presence of inflammation by the body’s autonomic nervous system [84].
…inflammation is also regulated in a complex manner by its antagonist, the sympathetic nervous system [86]. Its activation leads, among others, to the release of catecholamines, such as norepinephrine and epinephrine…”
It’s far more than just the gut, of course, but the GI component should never be overlooked.
“The application of the FMM identifies the gene--environment interactions that facilitate the patients nodal points and corrects them with emphasis on personalized diet, nutrition, and lifestyle changes.”
“The importance of addressing the proximal causes of atrial fibrillation is recognized, yet frustration with the currently applied preventive measures is high…the functional medicine model (FMM), which identifies the proximal causes of atrial fibrillation at the level of gene-environment interaction.
The pathological processes leading to atrial fibrillation sustaining disorder have been elucidated in translational studies and are described as ‘nodal points.’ Examples are inflammation, oxidative stress, autoimmune mechanisms, and visceral adiposity. These same nodal points also cause disorder that results in atrial fibrillation-related complications and the development of atrial fibrillation-associated diseases. These nodal points vary from patient to patient and can be identified by careful evaluation of the patients clinical phenotype.”
Tachycardia (abnormally rapid heart rate)
Supraventricular tachycardia can occur with afib undergoing catheter ablation.
“The IgG fractions from patients with inappropriate sinus tachycardia exerted a positive chronotropic action with a high prevalence of anti-beta receptor antibodies (52%)…The IgG fractions from healthy volunteers did not contain antiautonomic receptor antibodies.
Our results suggest, for the first time, a link between inappropriate sinus tachycardia and circulating anti-beta adrenergic receptor antibodies... This finding offers new insight…potential therapeutic consequences.”