Risks of high cholesterol and benefits of statins misrepresented by unacceptable scientific behavior
Focus on relative risk coupled with insufficient disclosure of absolute in the reporting of randomized controlled trial outcomes misleads healthcare providers and the public to overestimate concerns about high cholesterol and the benefits of cholesterol-lowering therapy.
An important paper, Historical Review of the Use of Relative Risk Statistics in the Portrayal of the Purported Hazards of High LDL Cholesterol and the Benefits of Lipid-Lowering Therapy, recently published in the Cureus Journal of Medical Science presents how the use of statistics obscures the correct import of RCT (randomized controlled trial) outcomes, inflating the risk of having high cholesterol and the amount of benefit from treatment to lower cholesterol levels.
The disturbing problem of credibility in general
The authors note that skepticism over the credibility of much published research in all areas is warranted.
“Richard Horton, editor of the Lancet, expressed the opinion that “much of the scientific literature, perhaps half, may simply be untrue” [1]. A similar sentiment was expressed by John Ioannidis, professor of medicine, epidemiology, and population health at Stanford, who stated, “There is increasing concern that in modern research, false findings may be the majority or even the vast majority of published research” [2]. Marcia Angell, former editor of The New England Journal of Medicine (NEJM), disclosed, “It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines” [3]. The skepticism over the credibility of much of the published research may have occurred, in part, from decades of misleading presentations of research findings by clinical trial directors.”
In this paper, the authors focus on how a key aspect of data analysis has been used by trial directors to obscure the objective findings to promote their agenda:
“While these eminent leaders of medical establishments have lamented over a range of flaws in the conduct of medical research, we have focused on one aspect of data analysis that has been deployed by many trial directors to promote their agenda, rather than to present their findings in the most objective manner possible. Specifically, directors have deployed a statistical strategy that can amplify a modest benefit of drug treatment to appear as if the effect is of great clinical significance. This statistical strategy focuses on the use of relative risk (RR) reduction and the exclusion or minimization of absolute risk (AR) reduction, which are two different ways to express the same raw data.”
For example, if 2 per cent mortality occurs in the placebo group and 1 per cent in the treatment group, a 50 per cent relative mortality reduction sounds great, but the absolute mortality reduction that has actually occurred is only 1 per cent, which is clinically trivial.
“Skolbekken [5] also illustrated this issue…in his critique of how the benefits of cholesterol-lowering therapy had been portrayed. The following is our summary of the data from hypothetical studies in his research: In one randomized controlled trial (RCT), 2,000 people die out of 10,000 in a placebo group, and 1,000 people die out of 10,000 in a treated group, resulting in an AR reduction of 10% (1,000/10,000=10%). In another RCT, two people die out of 10,000 in a placebo group, and one person dies out of 10,000 in a treated group, resulting in an AR reduction of 0.01% (1/10,000=0.01%). Despite the vast difference in the ARs between the two studies (10% and 0.01%), in both, the RR reduction was 50% (1,000 is 50% of 2,000, and one is 50% of two).
Skolbekken asserted that the “real impact of treatment … can only be seen by also reviewing the absolute risk reduction.” He also noted that reporting only the relative risk gives “a more favourable impression of the effectiveness of a drug than absolute risk estimates.” Numerous investigators have emphasized the importance of this issue; surveys have shown that lay people, as well as healthcare providers, overestimate the benefit of a treatment, such as cholesterol reduction, when the findings are presented solely as the RR [6-16].”
Misleading presentation of the data in cholesterol-heart disease research
The authors conducted an in-depth analysis of the five landmark randomized controlled trials (RCTs) that assessed heart disease monitoring and prevention over the past four decades. These well known studies are, in 1984, “Lipid Research Clinics Coronary Primary Prevention Trial” (LRC-CPPT) [17,18]; 1986, “Multiple Risk Factor Intervention Trial” (MRFIT) [19]; 2008, “Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin” (JUPITER) [20]; 2017, “Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk” (FOURIER) [21]; and 2023, “Cholesterol Lowering via Bempedoic Acid [ECT1002], an ACL-Inhibiting Regimen” (CLEAR) [22].
“These five clinical trials have been selected because they played a key role in implicating high total serum cholesterol, in general, and low-density lipoprotein cholesterol (LDL-C), in particular, in causing heart disease and in promoting the pharmacological reduction of cholesterol to prevent coronary heart disease (CHD). Our review addresses how the findings in each of these clinical trials were presented to all audiences primarily in the RR format, which exaggerated the role of cholesterol in CHD and amplified the modest benefits of lipid reduction.”
For each of these famous studies the authors conduct a detailed analysis that discloses how the emphasis on RR obfuscates the true conclusions that should be drawn from the data. I’ll mention a few here.
Miscommunication of the benefits of a lipid-lowering therapy
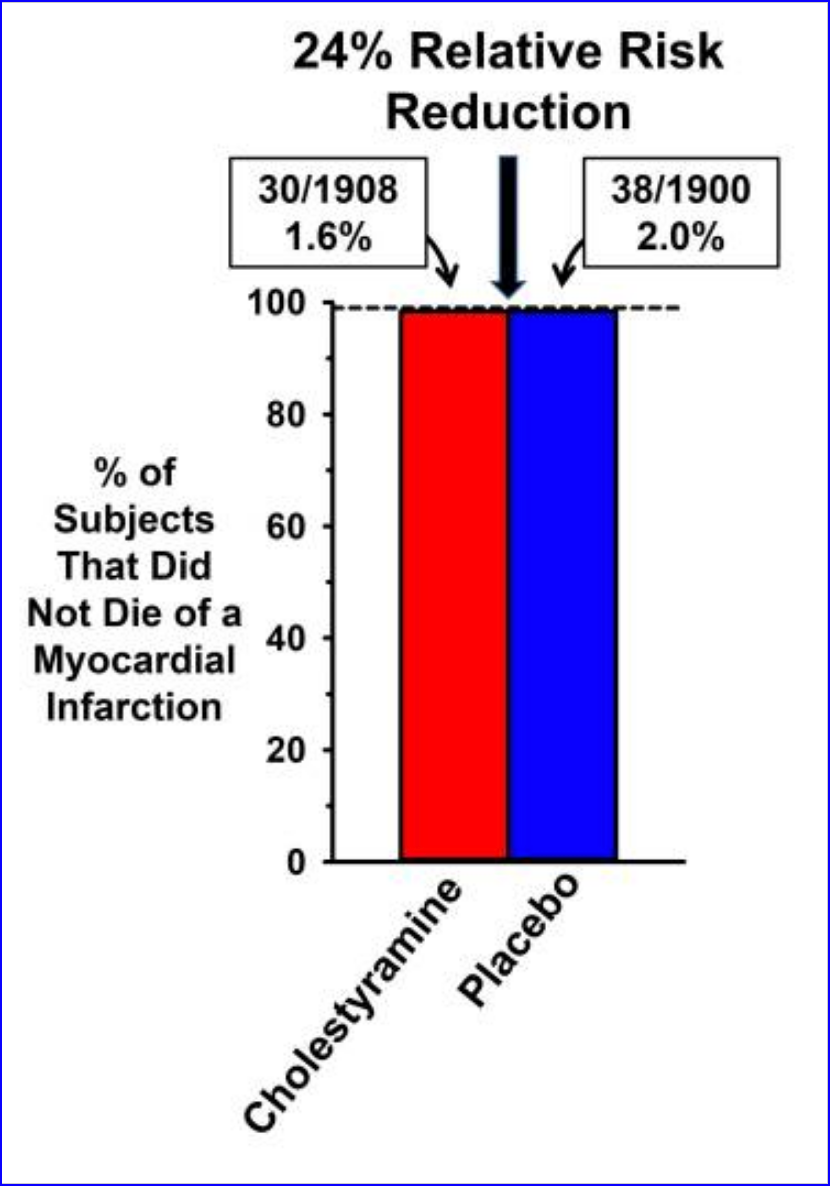
The LRC-CPPT (Lipid Research Clinics Coronary Primary Prevention Trial) addressed the hypothesis that “long-term reduction of serum cholesterol in hypercholesterolemic men initially free of CHD will lead to a lowered incidence of coronary heart disease”. Half a million middle-aged males with the highest (top 5%) cholesterol levels were put on a low-cholesterol diet for 7.4 years; about half were given a placebo and the remainder were given a bile-sequestering agent (cholestyramine), which reduced cholesterol levels. It’s amazing to see how this study established the dogma of cholesterol and heart disease on a misapprehension of the evidence.
“The authors reported that cholesterol reduction resulted in “a 24% reduction in definite CHD death and a 19% reduction in nonfatal myocardial infarction.”
There was widespread praise for the LRC-CPPT findings, as exemplified by an editorial in the British Medical Journal (BMJ), which stated, “At long last we have clear evidence that reducing very high plasma concentrations of cholesterol and low density lipoprotein (LDL) cholesterol lowers the incidence of coronary heart disease” [23]. Similar views were expressed in a lead article in the Medical Journal of Australia entitled “The lipid hypothesis is proven,” which declared “the incidence of death from definite CHD was reduced by 24% in the cholestyramine group” [24].”
In the same year in which the LRC-CPPT findings were published, the National Institutes of Health (NIH) convened a panel of experts on diet, cholesterol, and heart disease. The panel published a consensus statement, which was based, in large part, on the LRC-CPPT findings [25]. The NIH panel concluded, “It has been established beyond a reasonable doubt that lowering definitely elevated blood cholesterol levels … will reduce the risk of heart attacks caused by coronary heart disease. This has been demonstrated most conclusively in men with elevated blood cholesterol levels ….”
The NIH consensus panel and others failed to address the ensuing controversy over numerous irregularities that were found in the LRC-CPPT statistical methods. Most importantly, when absolute risk was considered instead of relative risk, the results become practically meaningless.
“Specifically, commentators accused the LRC-CPPT investigators of changing how they analyzed the data because the initial data analysis, based on their prespecified method paper [17], failed to support the hypothesis that cholesterol reduction would reduce CHD events or mortality….In Figure Figure1,1, we have illustrated the data in the original publication (from their Table 3) to highlight the difference between the magnitudes of the absolute risk reduction and the relative risk reduction.”
Comparison of absolute and relative risk benefits of cholesterol reduction in LRC-CPPT
The actual 0.4 percentage point absolute risk difference in heart disease mortality is clinically meaningless.
“How can the CHD mortality rate be reported as a 24% reduction in the publication, as well as in the medical journals that praised the findings, when the difference in CHD mortality between the two groups was only 0.4%? The explanation is that the AR mortality reduction of 0.4% was transformed into an RR reduction by dividing 0.4% by 2.0%, which resulted in a far more impressive 24% effect.”
“More objective assessments of flaws with the LRC-CPPT and the NIH consensus panel were provided at the time by two acknowledged experts. The first was from Dr. Thomas Chalmers of Mount Sinai Medical School, who commented, “I think they made an unconscionable exaggeration of all the data” [32]. The second was from Dr. George Mann, professor of biochemistry and medicine at Vanderbilt University Medical School [30]: “They have held repeated press conferences bragging about this cataclysmic breakthrough which the study directors claim shows that lowering cholesterol lowers the frequency of coronary disease. They have manipulated the data to reach the wrong conclusion. This is plain for any student of elementary statistics to see. The managers at NIH have used Madison Avenue hype to sell this failed trial in the way media people sell an underarm deodorant. The (NIH Consensus Panel) has failed to acknowledge that the LRC trial, like so many before it, is saying firmly and loudly ‘No, … the drug you generously tested for a pharmaceutical house does not work …’.”
MRFIT: Misrepresenting evidence to assert that small increases in cholesterol are harmful
The observational component of the MRFIT (Multiple Risk Factor Intervention Trial) assessed the hypothesis that there would be a positive association between serum cholesterol and CHD deaths in middle-aged males. It was promoted as demonstrating that small increment of total cholesterol, increased one’s risk of dying of CHD. It concluded that “of all CHD deaths, 46% were estimated to be excess deaths attributed (solely) to serum cholesterol 180 mg/dl or greater.”
“The data from Table 3 of the original publication are provided here as Figure Figure2,2, which illustrates the relation between serum cholesterol levels (in deciles) and CHD mortality. The same raw data generated the RR (red bars) and the AR (blue bars) for CHD death rates. The data presented as RR seem alarming, since they appear to strongly support the authors’ conclusion that small increments in cholesterol are associated with substantial increases in CHD risk. The graph illustrates that people with total cholesterol levels of 216 mg/dl, 246 mg/dl, and >290 mg/dl have two, three, and four times the risk, respectively, of dying of CHD, compared to people whose total cholesterol level is 150 mg/dl. This dramatic increase in CHD death supported the authors’ conclusion that cholesterol levels above 180 mg/dl “powerfully affects risk for the great majority of middle-aged American men.”
A proper analysis of absolute risk tells an entirely different story, where the difference in the rate of heart disease mortality between the lowest and highest levels of cholesterol was no more than 1%.
“A less alarming view of the MRFIT findings is provided by the overlapping AR data... The blue bars illustrate the AR of CHD in terms of the rate of survival in relation to cholesterol levels (the dashed line provides a visual guide at 99%, which indicates that at every level of cholesterol, the survival rate was approximately 99%). Thus, at the lowest level of cholesterol, CHD survival was 99.7%, and at the highest level of cholesterol, CHD survival was 98.7%. Thus, across the entire physiological range of cholesterol levels, the difference in the rate of six-year CHD mortality was only about one percentage point.”
Again, the authors did not mention the absolute risk which nullified their contention based on relative risk that elevated levels of cholesterol are implicated “in the causation of premature CHD.” This was instrumental in countering the trend that was building at the time that debunked the assertion that cholesterol causes heart disease.
“To put the MRFIT findings into historical perspective, by the 1980s, the hypothesis that cholesterol caused heart disease was falling out of favor. Clinical trials of different agents, such as corn oil [40], clofibrate [41], and cholestyramine [18], had all failed to demonstrate a significant clinical benefit of cholesterol reduction on coronary events and mortality. Had the trial directors focused on AR instead of RR, MRFIT should have served as the death knell for the cholesterol hypothesis. Cardiovascular research would have shifted its focus in subsequent decades on more traditional CHD risk factors, including smoking, stress, hypertension, hyperglycemia, and insulin resistance. Instead, MRFIT, as well as LRC-CPPT, provided the impetus for the expansion of cardiovascular disease (CVD) treatments to new approaches to reduce cholesterol, including the development of statins.”
The famous JUPITER trial purporting to show the benefit of statins
Statins have become so fervently advocated by many providers that to investigate the evidence invites castigation as a fear-mongering denial cultist.
“Statins reduce cholesterol levels by blocking the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-controlling enzyme in cholesterol synthesis. Statins have been considered so successful in preventing coronary events that William Roberts, MD, editor of the American Journal of Cardiology, described statins as “miracle drugs,” which “are to atherosclerosis what penicillin was to infectious diseases” [42]. Praise for statins has been so strong that critics have been labeled as members of a “statin denial cult” [43], who disseminate “fake medical news and fearmongering … through relentless attacks on statins” [44].
We suggest that statins are promoted as “miracle drugs” largely because statin advocates have often publicized their RR reduction while failing to highlight their modest AR reduction. Moreover, the adverse effects of statins have been minimized or ignored entirely. Diamond and Ravnskov [45] provided a critique of this strategy in their assessment of clinical trial outcomes for statin treatment in primary and secondary prevention of CHD.”
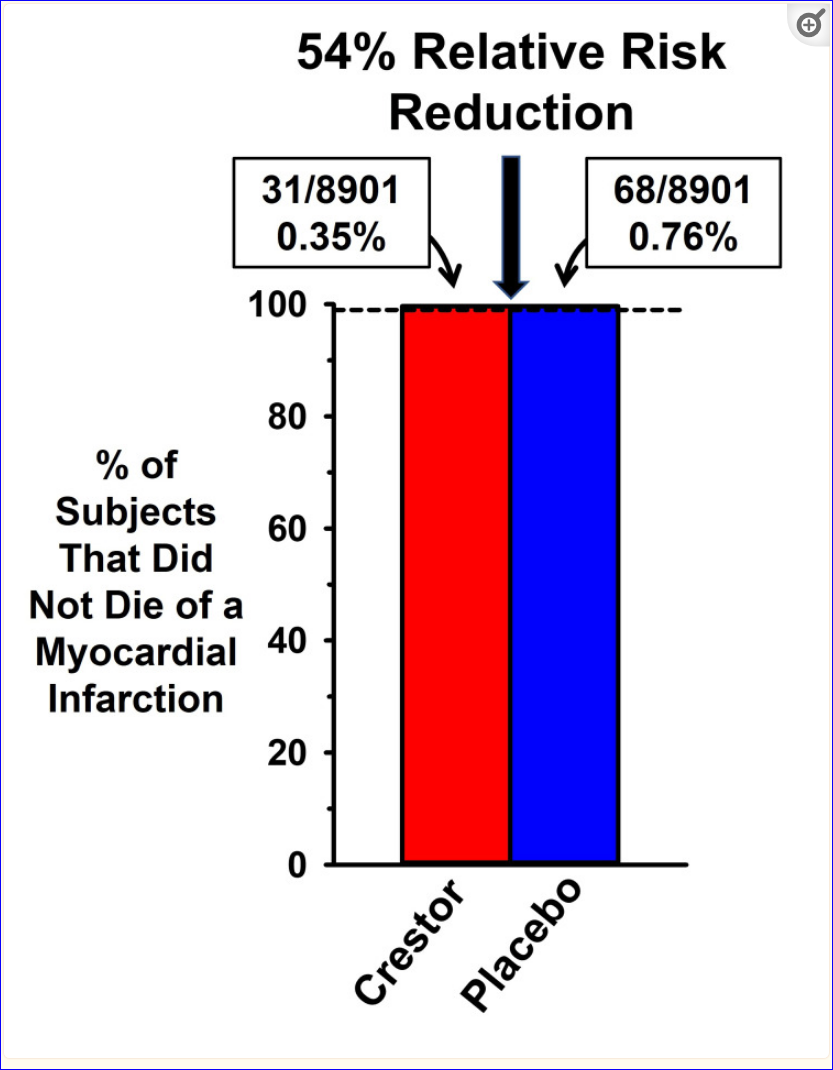
JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) was a landmark study that was the major ‘game-changer’ for the use of statins in cardiovascular prevention. The intent was to prove whether a statin (rosuvastatin aka Crestor) would reduce vascular events in people without hyperlipidemia. At that time, the reference range for cholesterol was much higher.
“JUPITER was a landmark trial with findings that were embraced by many healthcare providers because it appeared to demonstrate substantial benefits for people without high cholesterol and with a low risk for CHD. In two representative comments on JUPITER outcomes, Dr. Steven E. Nissen, director of cardiology at the Cleveland Clinic, proclaimed, “It’s a breathtaking study. It’s a blockbuster. It’s absolutely paradigm-shifting,” and Dr. W. Douglas Weaver, president of the American College of Cardiology, was equally emphatic: “This takes prevention to a whole new level. Yesterday you would not have used a statin for a patient whose cholesterol was normal. Today you will” [46].”
It appeared to be so successful that it was stopped prematurely. But as with the two previous trials, the results were misrepresented by emphasizing RR (relative risk) benefit with “little to no mention of the AR (absolute risk)".”
"This data in Table 3 of the original publication, which illustrates the great disparity between how the AR and RR represent the magnitude of rosuvastatin effects on coronary events.
The dashed line at 99% is a visual aid to illustrate that over 99% of rosuvastatin (Crestor) and placebo subjects did not suffer from a fatal myocardial infarction (MI). If more than 99% of all subjects did not die as a result of an MI, how was it that the authors could claim that rosuvastatin reduced the rate of a fatal MI by 54%? The 0.41 percentage point absolute risk difference in MI mortality between the Crestor and placebo groups was converted into a 54% relative risk reduction in events.”
However, the extensive adverse effects were reported as AR rather than RR, making them appear far smaller!
“Although the AR reduction with rosuvastatin treatment was modest, a small benefit could be of value if statins had very few and only minor adverse effects. However, the adverse effects of statins are extensive [45,48-59], including an increased risk of new-onset type 2 diabetes [20,60-65], an increase in fasting blood glucose in patients with and without diabetes [66], mitochondrial dysfunction [67-69], tendinopathy [70], myopathy [71,72], acute kidney injury/renal failure [73-75], and cognitive deficits [54,76-83].
The JUPITER trial documented a significant increase in the incidence of new-onset diabetes with rosuvastatin treatment compared to placebo (their Table 4). In reporting this adverse effect of statins, the authors presented the data only in terms of its AR, without transforming it into the RR. According to Gigerenzer et al. [84], the portrayal of the RR without including the AR provides “incomplete and misleading information,” which they referred to as “mismatched framing.”
If the authors had used the RR, they would have reported a 25% increase in new-onset diabetes on the statin. The trial director described it as a “play of chance”.
“However, the significant increase in the incidence of diabetes with statin treatment has been reported in numerous subsequent publications [60-64,85], including an RCT that characterized the mechanistic basis as to how statins increase the susceptibility of users to develop diabetes [65]. This finding is relevant to why RCTs have demonstrated an increase in fasting blood glucose in patients with and without diabetes [66], as well as a meta-analysis that suggested females are more susceptible than males to develop type 2 diabetes with statin treatment [60].”
When the JUPITER trial findings were first reported, the manipulation did not go unnoticed:
“The findings of the JUPITER trial were subject to widespread criticism, similar to the controversy generated in response to LRC-CPPT. According to Curtiss and Fairman [86], “Criticism of the JUPITER trial results began immediately” and developed into “an avalanche” of published critiques [87-90]. Criticisms were raised at multiple levels, including questionable justification for terminating JUPITER in less than two years despite the plan to run the trial for four years. There were also concerns that the premature termination was influenced by the pharmaceutical company sponsoring the trial. There was also criticism of the efforts by the authors to downplay the increased incidence of diabetes with drug treatment. It is noteworthy that Serebruany [91] asserted that “the medical community does not uniformly accept the results of the JUPITER trial, engaging in heavy, somewhat personal debates over the validity of the JUPITER findings” including “commercial interests of the principal investigator” and a “fundamental problem with trial integrity.”
The biggest issue was how emphasizing the RR and ignoring the AR made the statin intervention appear, falsely, beneficial:
“Critics commented that the study outcomes emphasized the RR and ignored the AR reduction. For example, Vaccarino et al. [90] expressed concerns regarding the lack of attention to the modest AR benefit in JUPITER. These authors stated that the trial was terminated prematurely based on the 44% RR reduction in events, but they echoed the concerns of others by stating, “what really matters for dictating changes in clinical practice is the absolute risk reduction…Despite the appearance of an impressive 54% RR, the modest AR led Vaccarino et al. to conclude that “the treatment benefits achieved in the JUPITER trial are not large enough to advocate an expansion in the clinical indications for statins.”
But the FDA granted approval for prevention anyway.
Cholesterol-lowering benefits misconstrued in recent research
The FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial reported that the antibody-based drug evolocumab reduced LDL-C by 59%, but the benefit was again misrepresented by mentioning only the RR and not the AR.
“According to Sabatine, “Evolocumab reduced the risk of cardiovascular events, a 15% reduction in the primary endpoint, a 20% reduction in the risk of cardiovascular death, MI or stroke” [92]. The 15%-20% benefits of evolocumab treatment were highlighted, as well, in the opening paragraph of the Discussion section of the publication [21].
Sabatine’s approach to data presentation followed the now routine practice of mentioning only the RR, without including the AR. The impact of the FOURIER findings may have been less impressive had the reduction in the risk of the composite of cardiovascular death, MI, or stroke been expressed as the AR, which was only a 1.5 percentage point difference between treatment and placebo (5.9% versus 7.4%).”
Moreover, there was no statistically significant difference in death due to any cardiovascular condition and no effect on all-cause mortality.
And it’s a similar story for the recent CLEAR (Cholesterol Lowering via Bempedoic Acid) trial which tested bempedoic acid (reduces hepatic cholesterol synthesis and raises LDL receptor expression, thereby increasing the clearance of LDL cholesterol from the circulation) on statin-intolerant patients. Although it reduced LDL-C levels by 26.1%, the actual benefit was nil and outweighed by side effects:
“The group-administered bempedoic acid exhibited a 26.1% reduction in LDL-C levels (compared to a 10.6% reduction in the placebo group). The AR reduction with bempedoic acid for combined major coronary adverse events was 1.5 percentage points (13.3% versus 11.7%), with no benefit in fatal or nonfatal stroke or death from any cause, including cardiovascular causes. Bempedoic acid treatment produced adverse effects not seen with statins, including a significant increase in hyperuricemia (10.9% versus 5.6%), gout (3.1% versus 2.1%) and cholelithiasis (gall stones) (2.2% versus 1.2%), renal impairment (11.5% versus. 8.6%), and elevated hepatic enzyme level (4.5% versus. 3.0%).”
And as usual now, in discussion the RR was used to exaggerate benefit while the AR was brought up only to downplay the side effects:
“In a discussion of the CLEAR outcomes, Dr. Ann Marie Navar did not mention the AR 1.6 percentage point difference in the benefits of bempedoic acid treatment. Instead, she presented the benefits of bempedoic acid in terms of the RR data, by stating that there was a 13% RR reduction in events [95]. In contrast, when Dr. Navar addressed the adverse effects of the treatment, she mentioned only the AR data, stating that there was only a 1% increase in the incidence of gout and cholelithiasis (the formation of gallstones), with no mention of the increased incidence of renal impairment, elevated hepatic enzyme level, and near doubling of the incidence of hyperuricemia with bempedoic acid treatment versus placebo. Gigerenzer et al. [84] considered this form of data presentation to be the second “sin” against transparent reporting, that is, deliberately reporting benefits as relative risk reductions while reporting harms as absolute risk increases.”
Healthcare providers misled by exclusive presentation of data as RR
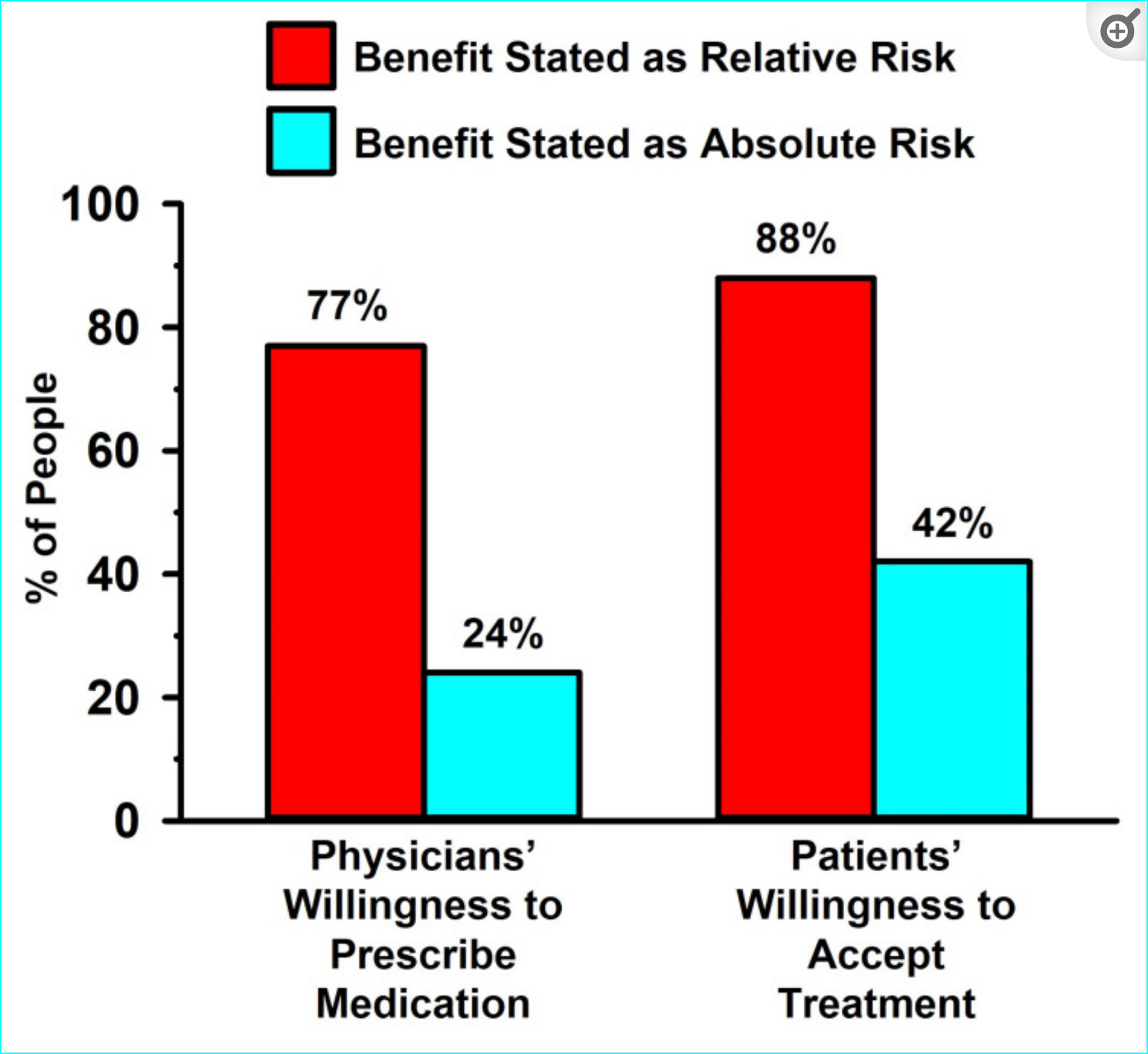
Healthcare providers are far more likely to prescribe lipid-lowering medication when informed only of the RR (relative risk) and way less likely to prescribe when the AR (absolute risk) has been communicated.
“As an example of how healthcare providers can be misled by an exclusive presentation of data as RR, Bucher et al. [97] demonstrated that physicians were more inclined to prescribe cholesterol-lowering medication for hypercholesterolemia when the trial results for identical endpoints were expressed as RR reduction, compared to AR reduction. Sackett and Cook [98] commented on this finding by stating that “restricting the reporting of efficacy to just relative risk reductions can lead to greater - and at times - excessive zeal in decisions about treatment for patients with low susceptibilities.”
Influence of data presented as relative risk versus absolute risk in patient and physician decision-making:
“When the lipid-lowering effects were presented as RR, more than three-quarters of the clinicians were amenable to prescribing the medication, and even more of the patients were amenable to taking the medication. By contrast, when the data were presented as AR, only a quarter of the physicians were amenable to prescribing the medication, and less than half of the patients were amenable to taking the medication.”
For clinical decision making, the data reported in absolute terms should always be the benchmark:
“Stegenga [103] emphasized that “Effectiveness always should be measured and reported in absolute terms (using measures such as ‘absolute risk reduction’)” and he further stated that “people’s comparative understanding of relative versus absolute outcome measures is dubious. Relative measures … fundamentally mislead patients into overestimating effectiveness.” Nevertheless, peer-reviewed medical publications and the media exhibit a high prevalence of bias toward reporting the RR and ignoring the AR.”
“…this strategy by clinical trial investigators to amplify the appearance of the magnitude of their intervention is unacceptable scientific behavior. We concur with Gigerenzer et al. [84] that journal editors should enforce transparent reporting of data, including the requirement that papers include the AR and RR in the abstracts. In addition, an education on the miscommunication of risk and benefits in research should be included in the training of healthcare workers and students of public health.”
Distraction from important causes of vascular disease—and the benefits of LDL-C
The atherogenic modifications in plasma, including glycation—binding with excess glucose (hyperglycemia) which is associated with heightened inflammation, insulin resistance with elevated levels of insulin that are destructive to the glycocalyx (protective sheath of the inner lining of the blood vessel), that permits vascular inflammation, and atherogenic modifications of sdLDL leading to the formation of vulnerable plaque are then typically ignored. Moreover, it obscures the importance of LDL-C for health.
“…the exaggeration of the putative harms caused by elevated LDL-C [104] and the benefits of lipid-lowering medications has detracted from an appreciation of the physiological relevance of elevated levels of LDL-C in optimal health. For example, LDL-C is an important component of the immune system [105-107]. Chronically elevated LDL-C levels may enhance aspects of immune functioning, which is potentially relevant to the finding that elderly people with familial hypercholesterolemia (FH) have lower rates of mortality from cancer and infection compared to the general population [108-110].”
Moreover, we know that LDL-C is a very poor predictor of MACE (major adverse cardiovascular events) and more than half of heart attacks occur with low LDL-C.
“The importance of LDL-C to overall health may explain why LDL-C is such a poor marker of risk for CVD [34-38], as well as cardiovascular and all-cause mortality [39]. Coronary artery calcification (CAC), in contrast to LDL-C, is the single best predictor of future fatal and nonfatal coronary events [111-121]. Moreover, among those with genetically confirmed FH [familial hypercholesterolemia], approximately half showed no detectable CAC and had a favorable prognosis, despite significantly elevated LDL-C levels [122]. These observations help to explain why FH individuals do not face an increased risk of CVD mortality with advanced age, as well as the greater longevity of people in the general population with high LDL-C, compared to those with low LDL-C [39].”
And this tracks with the diminished benefits of statins when examining the correct metric, AR (absolute risk):
“Finally, the overestimation of the involvement of LDL-C in producing CHD based on RR statistics is confirmed by the modest AR benefits of statins…This finding was quantified recently by Byrne et al. [132] in a systematic review and meta-analysis of statin RCTs. These investigators reported that the AR reduction of statins was only 0.6% for all-cause mortality, 0.7% for MI, and 0.3% for stroke and 0.9%, 2.2%, and 0.7%, respectively, in primary and secondary prevention…This finding of limited benefits of statins is further confirmed by the work of Kristensen et al. [133] who reported that overall, statin treatment delayed death in primary and secondary prevention trials by only 3.2 and 4.1 days, respectively. These findings support the conclusions of Byrne et al. [132] that “when considering the ARR of statins, the benefits are quite modest, and most trial participants who took statins derived no clinical benefit.”
This all makes it fair to say that LDL-C has simply gotten a ‘bad rap’:
“Hence, the pejorative view of LDL-C as the “bad cholesterol,” which has been perpetuated by the disproportionate emphasis on RR statistics, is not supported by a balanced review of the literature. The characteristic of this perspective is the opinion that “evidence falsifying the hypothesis that LDL drives atherosclerosis has been largely ignored” [134], and the opinion of three cardiologists that “LDL cholesterol risk has been exaggerated” [135] (see also Ravnskov et al. [34] and Diamond et al. [136] for related reviews and discussion).”
Other scientists have attempted to spotlight this problem
The authors of this paper (‘Historical Review of the Use of Relative Risk Statistics in the Portrayal of the Purported Hazards of High LDL Cholesterol and the Benefits of Lipid-Lowering Therapy’) state:
“We are not the first to object to the preferential use of RR, without sufficient attention to AR, in clinical trial reporting. For decades, academicians have deplored the strategy taken by some clinicians to promote their findings by emphasizing RR to the exclusion of reporting the AR [6-16]. In one example, Gigerenzer et al. [84] considered this form of data presentation to be “the first ‘sin’ against transparent reporting.”
Another study published last year in JAMA Internal Medicine also scrutinized this problem with regard to cholesterol and statins in a systematic review and meta-analysis and came to the same conclusions:
Another study published last year in JAMA (Journal of the American Medical Association) Internal Medicine also examined the same problem of misrepresenting data with regard to cholesterol and statins in a systematic review and meta-analysis, Evaluating the Association Between Low-Density Lipoprotein Cholesterol Reduction and Relative and Absolute Effects of Statin Treatment, and came to the same conclusions.
They too noted that the association between statin-induced reduction in low-density lipoprotein cholesterol (LDL-C) levels and the absolute risk reduction of outcomes, such as all-cause mortality, heart attacks, and strokes was not being made clear. So they set about to…
“…assess the association between absolute reductions in LDL-C levels with treatment with statin therapy and all-cause mortality, myocardial infarction, and stroke to facilitate shared decision-making between clinicians and patients and inform clinical guidelines and policy.”
They included twenty-one large randomized clinical trials that examined the effectiveness of statins in reducing total mortality and cardiovascular outcomes, comparing treatment with statins and reduction of LDL-C to placebo (and no change in LDL-C). Three independent reviewers assessed the quality of the evidence, extracted the data, resolved their decisions by consensus, conducted the meta-analyses. What did they conclude?
The results of this meta-analysis suggest that the absolute risk reductions of treatment with statins in terms of all-cause mortality, myocardial infarction, and stroke are modest compared with the relative risk reductions, and the presence of significant heterogeneity reduces the certainty of the evidence. A conclusive association between absolute reductions in LDL-C levels and individual clinical outcomes was not established, and these findings underscore the importance of discussing absolute risk reductions when making informed clinical decisions with individual patients.
Stated succinctly:
“The study results suggest that the absolute benefits of statins are modest, may not be strongly mediated through the degree of LDL-C reduction, and should be communicated to patients as part of informed clinical decision-making as well as to inform clinical guidelines and policy…
The transparent communication of RRR (relative risk reduction) and ARR (absolute risk reduction) by clinicians, as well as the potential for harm, to their patients may lead to more informed decision-making about the true benefits and risks of statins.31 In addition, our findings have implications for future clinical guideline development and for policy makers and payers considering the opportunity cost of statin therapy.”
Bottom line
The authors of the first paper cited, ‘Historical Review of the Use of Relative Risk Statistics in the Portrayal of the Purported Hazards of High LDL Cholesterol and the Benefits of Lipid-Lowering Therapy’, conclude:
“We have reviewed the findings of five landmark clinical trials conducted over the past four decades that have supported the current consensus that high LDL cholesterol causes CVD and that the pharmacological reduction of LDL produces substantial CVD benefits. Our assessment of these trials demonstrates that the association of cholesterol with CVD is far more modest than has been portrayed. We also assert that the promotion of statins as “miracle drugs” has been based on an emphasis on their RR reduction, which amplifies the appearance of their modest AR benefits.”
Sadly…
“The biased approach to data analysis in the five trials we have reviewed is representative of the now common practice of highlighting RR over AR in data presentation and in the media, which has been referred to as a “miscommunication of risk.” A consequence of this biased approach to the reporting of CVD clinical trial findings explains in large part why healthcare providers and the public have overestimated the purported hazards of high cholesterol and the benefits of cholesterol reduction.”
When will this scientifically unacceptable and misleading practice change?
In conclusion, for the past four decades, academics have repeatedly asserted that the portrayal of clinical trial findings as the RR, while disregarding the AR, is a deceptive practice. In the cardiovascular disease field, a consequence of this strategy has resulted in the exaggerated appearance of the purported hazards of high cholesterol and an amplification of the magnitude of benefits of cholesterol-lowering medications. This strategy appears to have been deployed for the first time with the publication of the LRC-CPPT trial in 1984 but has now become commonplace. To counter this trend of scientific misconduct, we assert that publications and media reports of clinical trial findings should always portray the benefits, as well as harms, of interventions in terms of both absolute and relative risks.
Readers may also be interested in this study in the journal Circulation showing evidence that higher levels of cholesterol are associated with less atrial fibrillation in their female cohort.