Telomere dysfunction without shortening also drives immunosenescence
Measuring telomere length as a metric for biological age and likely burden of senescent cells is shadowed by the new finding that cells can lose the ability to reproduce healthy clones even though telomere length remains the same.
Research having great clinical significance for all diseases for which aging is a risk factor titled Telomeric 8-oxo-guanine drives rapid premature senescence in the absence of telomere shortening has recently been published in Nature Structural & Molecular Biology. It identifies oxidative damage to DNA as the culprit and is associated with a biomarker we can readily measure in a urine test.
The authors point out how progressive telomere shortening over many cycles of division functions to prevent the production of abnormal cells but also results in the accumulation of senescent cells that contribute to many diseases, the so-called “zombie cells” that don’t function properly but actively secrete chemicals that drive non-purposeful inflammation and damage healthy cells:
“Mammalian telomeres consist of 5′-TTAGGG-3′ arrays bound by shelterin—a protein complex that remodels the chromosome end to suppress inappropriate recognition by DNA damage response (DDR) signaling1. Progressive telomere shortening with cell division activates the DDR and triggers ‘replicative senescence’ characterized by cell cycle arrest and phenotypic changes2,3. Thus, telomeres act as potent tumor suppressors by limiting proliferation4. However, senescent cells accumulate with age and contribute to numerous ageing-related pathologies by compromising regenerative capacity and secreting inflammatory cytokines, chemokines and proteases that promote inflammation and alter the tissue microenvironment5. The microenvironment becomes more permissive for tumor growth and, thus, paradoxically senescence can also promote tumorigenesis, metastasis or immunosuppression6,7,8. Telomere dysfunction in premalignant cells with compromised DDR signaling can cause chromosomal fusions and instability, which drive carcinogenesis9,10. Thus, telomere function and integrity are critical for genome stability, cellular function and organism health.”
But it is now shown by their research that healthy cells can be triggered to become senescent not just by telomere shortening but as a result of oxidative stress.
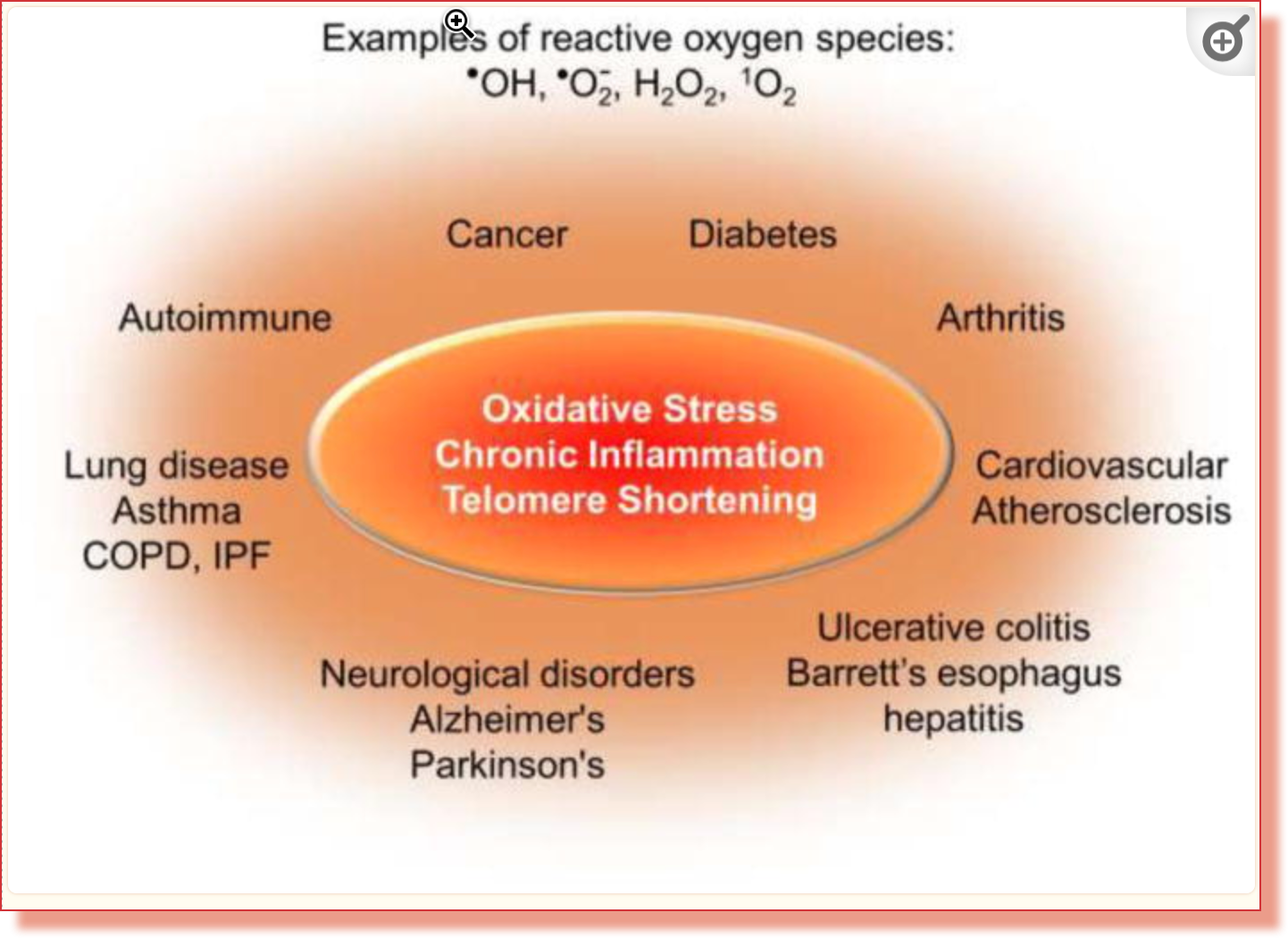
Oxidative stress and telomere dysfunction
Guanine is the most vulnerable to oxidative stress of the four main nucleobases that make the nucleic acids DNA and RNA. Damage due to oxidative stress and chronic inflammation may be more of a problem for telomere replication than shortening.
“Oxidative stress, which occurs when reactive oxygen species (ROS) exceed antioxidants, can promote senescence, degenerative diseases and aging13,14,15. Guanine is the base most susceptible to oxidation, and TTAGGG repeats are preferred sites for production of the common oxidative lesion 8oxoG16,17. These data led to a model, proposed around 20 years ago, that oxidative modification to telomeric bases may contribute more to telomere loss and telomere-driven senescence than the end-replication problem18. ROS-induced damage was also proposed to explain telomere dysfunction arising in low-proliferative tissues, such as lung and heart, independently of telomere length changes19,20,21,22,23.”
8-oxo-guanine (8oxoG)
8oxoG due to oxidation is a pivotal agent involved in the telomere damage resulting in premature senescence. Moreover, it does this to healthy cells very rapidly.
O2 is a main contributor of UVA radiation-induced oxidation reactions, arises from inflammation, lipoxygenases and dioxygenases, and forms primarily 8oxoG when reacting with DNA30,31. The physiological importance of 8oxoG is underscored by the evolution of three dedicated enzymes that specifically recognize 8oxoG in various contexts to enable repair and prevent mutations32,33.
…acute production of 8oxoG in telomeres is sufficient to rapidly impair growth of nondiseased human fibroblasts and epithelial cells. Using our chemoptogenetic tool, we show a single 5 min production of telomeric 8oxoG induced numerous hallmarks of cellular senescence within 4 days…
…We demonstrate the mechanism is by 8oxoG provoking replication stress-induced DDR activation and telomere fragility, rather than by accelerating telomere losses or shortening. Our data reveal a new mechanism of rapid telomere-driven senescence triggered by a common oxidative stress-induced base lesion that is distinct from ‘replicative senescence,’ and has important implications for cellular aging linked to oxidative stress.
8oxoG disrupts telomere replication without causing shortening
The authors’ proof that the telomere replication resulting in immunosenescence can occur without shortening of the telomere is a crucial point because, until now, the only metric used by all lab tests (that I know of) that attempt to measure biological age related to telomeres is their length.
“Telomeric 8oxoG triggered senescence by 4 days, a time frame typically insufficient to observe notable telomere shortening, particularly in telomerase-proficient cells (normally requires weeks). Analysis of telomere restriction fragments revealed no change in the bulk telomere lengths 4 days after DL (Extended Data Fig. 8a). Since a few critically short telomeres can promote senescence52, we used the telomere shortest length assay (TeSLA) to visualize the shortest individual telomeres. Although we detected individual telomeres much shorter than in the bulk population, DL did not increase the percentage of short or truncated telomeres (Extended Data Fig. 8b). Thus, telomere shortening does not need to precede oxidative stress-induced senescence.”
Implications for general health and longevity
Their research describes a process that happens throughout the body, importantly including white blood cells that results in the immunosenecence associated with so many chronic diseases for which aging is such a large risk factor.
“The key finding from our study that a small, nondistorting oxidative base lesion within telomeres is sufficient to induce premature senescence in the absence of telomere shortening is surprising, but provides a mechanistic explanation for telomere dysfunction foci arising in vivo in various contexts27.
Importantly, our study demonstrates premature senescence in primary and nondiseased human cells following induction of a common, physiological oxidative DNA lesion targeted to the telomere. Oxidative stress is a ubiquitous source of DNA damage that humans experience due to endogenous metabolism and inflammation, exogenous environmental sources as well as life-stress, and 8oxoG levels are elevated in aged humans79,80. Our results highlight the importance of understanding how and where this DNA lesion arises within human genomes, since its presence at telomeres alone is sufficient to rapidly advance cellular aging. While other oxidative lesions may also contribute to telomere instability, 8oxoG is among the most abundant. In summary, our studies reveal a new mechanism of telomere-driven senescence linked to oxidative stress.”
In earlier research, prior to the recognition of the role of 8oxoG in telomere attrition that can occur without shortening, the authors summarized some of the effects of senescence due to oxidative damage diminished telomere length:
Research that led up to the most recent findings highlighted in this post also includes Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis, The origin of oxidized guanine resolves the puzzle of oxidation-induced telomere-length alterations, and Oxidative guanine base damage regulates human telomerase activity. The main study described in this post was also reviewed under the title ‘Oxidative Damage Study Reveals Secrets of “Zombie” Cells’ published in Technology Networks Proteomics and Metabolomics.
The authors of the main paper conclude:
“Importantly, our study demonstrates premature senescence in primary and nondiseased human cells following induction of a common, physiological oxidative DNA lesion targeted to the telomere. Oxidative stress is a ubiquitous source of DNA damage that humans experience due to endogenous metabolism and inflammation, exogenous environmental sources as well as life-stress, and 8oxoG levels are elevated in aged humans79,80. Our results highlight the importance of understanding how and where this DNA lesion arises within human genomes, since its presence at telomeres alone is sufficient to rapidly advance cellular aging. While other oxidative lesions may also contribute to telomere instability, 8oxoG is among the most abundant. In summary, our studies reveal a new mechanism of telomere-driven senescence linked to oxidative stress.”
Clinical Note
Exhaustion of the ability of the best adapted immune cells to replicate clones resulting in the increase of ‘zombie’ T cells can occur due to the whittling down of telomeres by repeated cycles of expansion in response to immune challenges, including new infections and the ‘blooming’ out of the remnants of earlier infections as a consequence of disadvantageous immune polarization. We see in the main paper discussed here how it can also occur due to oxidative damage. A direct measurement of immunosenescence by T cell populations is done by the UCLA Immunoassay noted below. This is associated with telomeres rendered dysfunction either by replicative exhaustion or oxidative damage.
Oxidative damage to DNA associated with 8oxoG can be assessed by measuring 8-hydroxy-2'-deoxyguanosine (8-OHdG) in urine, available to clinicians from Genova and Doctor’s Data laboratories.
In personal communication with author Patricia L. Opresko, PhD, Professor of Environmental and Occupational Health at the University of Pittsburgh Graduate School of Public Health and Co-Leader of the Genome Stability Program at the UPMC Hillman Cancer Center, I asked her opinion about using 8-OHdG as a metric for the telomere-driven senescence documented by their research. She states:
“There is an established correlation between oxidative stress and telomere dysfunction, but systematic studies of potential correlations between 8-OHdG levels and telomere dysfunction still need to be done. Certainly, the findings in our recent paper would predict that higher levels of 8-OHdG in general should correlate with increased telomere dysfunction and senescence.”
The most direct measurement of immunosenescence characterized by the expansion of “zombie” CD28- T cells and diminished CD95- naive T cells is available by special arrangement with the UCLA Immune Assessment Core laboratory as detailed in the recent post Immunosenescence—immune deterioration associated with age—and social stress.
My current approach to assessment is to minimize costs to patients by deferring measurement of telomere length due to this new proof that telomeres can be dysfunctional without a change in length. Instead, while the UCLA Immunoassay documents the degree of immunosenescence, the test for 8-hydroxy-2'-deoxyguanosine (8-OHdG) is a metric for the amount of oxidative stress that is contributing. This may prove to allow an extrapolation to telomere length. A telomere length assay can still be added if further clarification is required. This is, of course, done in the context of the systems biology perspective that inventories the comprehensive range of contributing causes characteristic of the functional medicine clinical model.